Neuralink Seeks Second Participant for Brain Implant Trial
Renowned entrepreneur Elon Musk, known for leading Tesla, SpaceX, and Neuralink, announced through a tweet that Neuralink is actively searching for a second participant to engage in a brain implant trial. In his characteristically creative style, Musk described the implant as a “telepathy cybernetic brain implant that allows you to control your phone and computer simply by thinking.”
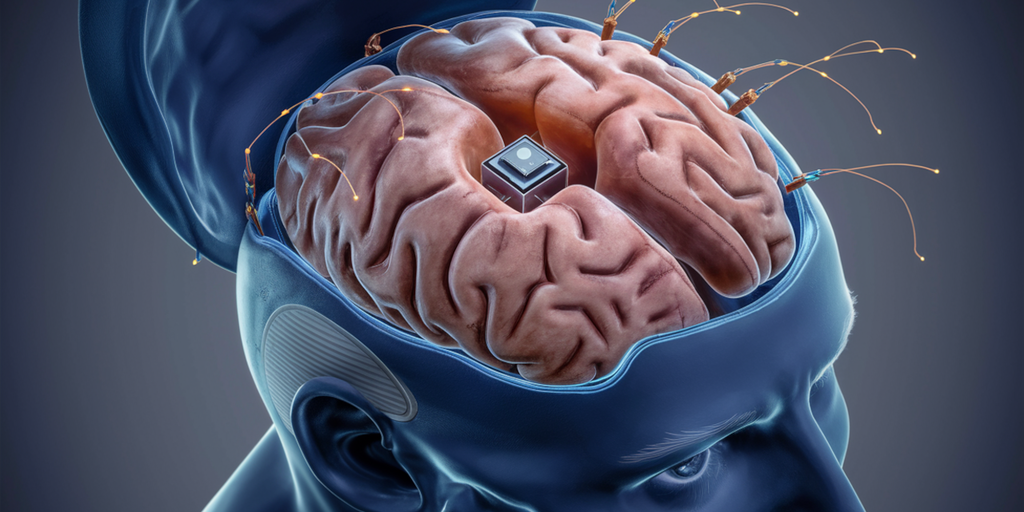
Neuralink, a company founded by Musk, is dedicated to developing brain-computer interfaces (BCIs). These interfaces aim to empower individuals with conditions like paralysis to manipulate digital devices using their thoughts exclusively.
Details of the Trial
While Musk did not delve into specifics regarding the trial in his announcement, and its validity was left somewhat ambiguous, Neuralink has not shared any related information on its official social media channels. Nonetheless, the company’s recent PRIME study update invites interested individuals to consider becoming test subjects for its clinical trial.
The PRIME study, standing for “Pilot Research Implant for Medical Experimentation,” serves as Neuralink’s effort to assess the safety and initial functionality of its fully implantable, wireless BCI device—the N1 chip. The implantation process involves the placement of thousands of minuscule threads into the brain.
Prior Controversies
Notably, Neuralink has faced controversies in the past, including claims from experts regarding the fatalities of over a thousand animals in animal trials—a claim that Musk disputes. Concerns have also been raised about Neuralink’s alleged failure to provide necessary documentation related to the calibration and maintenance of its vital signs monitoring system.
In January, Neuralink achieved a milestone by successfully implanting the device into the brain of its initial patient, Noland Arbaugh. Arbaugh, who suffers from paralysis extending from the shoulders down following a 2016 diving incident, reported substantial improvements with the implant. He is now able to engage in activities such as playing video games, browsing the internet, and manipulating a computer cursor using only his thoughts.
Recent Developments
Recently, Neuralink disclosed an issue during the first human trial where the tiny wires within the brain of the patient shifted out of position, resulting in fewer electrodes available for measuring brain signals. Nevertheless, the company managed to rectify this setback by implementing adjustments, including algorithm refinements to enhance sensitivity.
Competing alternatives from other companies, such as Precision Neuroscience, are emerging to address potential risks associated with brain implants. These alternatives propose less invasive methods, like coating the brain with an electrical blanket to monitor activity.
Eligibility and Application Process
The criteria for eligibility in the Neuralink trial include individuals located in the United States or Canada, aged 18 and above, experiencing quadriplegia, paraplegia, vision or hearing loss, speech impairment, or significant limb amputation. Prospective participants meeting these conditions can apply by visiting Neuralink’s designated Patient Registry.
Image/Photo credit: source url